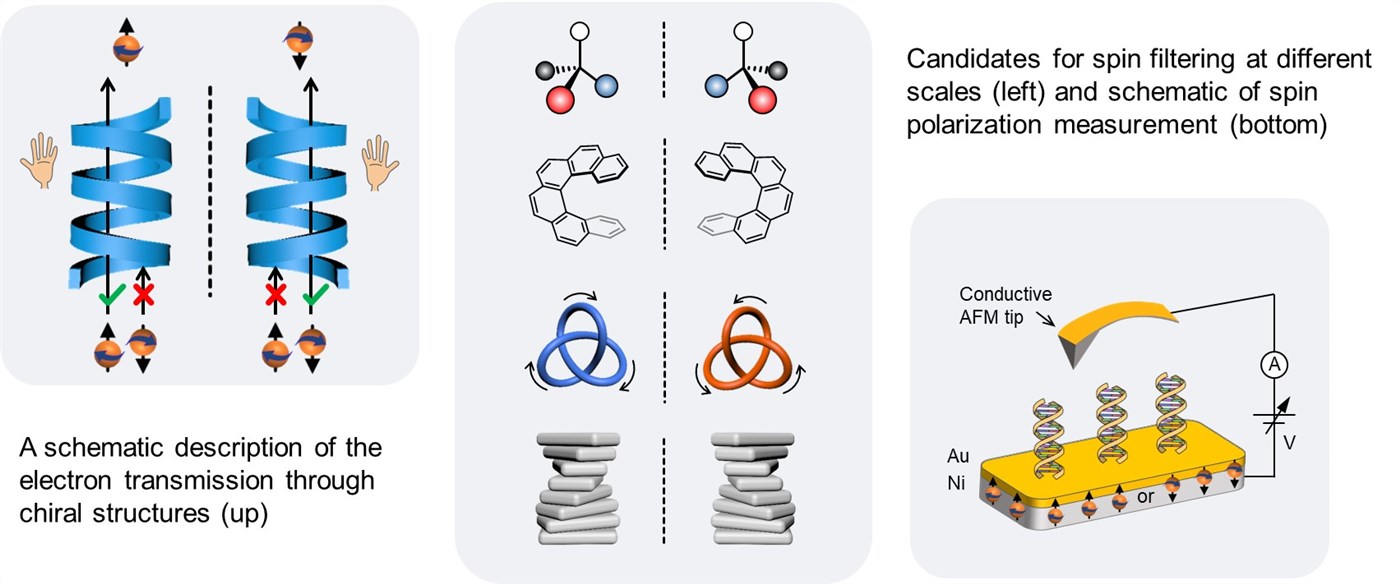
Why life is chiral has puzzled scientists for well over a century. Many essential biological molecules exist only in one of two possible mirror-image enantiomers, like L-amino acid and D-sugar. But the origin of this single-handedness (namely homochirality) has not been unravelled. The “conventional wisdom” is that chirality serves as a structural motif to place chemical functionalities in defined positions and orientations that enable biologically relevant functions. The recently discovered chiral-induced spin selectivity (CISS) effect implies that chiral molecules (or moieties) have a preferred orientation of the electron spin in the molecular frame after charge polarization. This has incredibly interesting applications since it gives us a new tool to control spins at the molecular level and room temperature. Our research focuses on 1) exploring the fundamental mechanisms of this effect underpinning experimental observations, with a particular focus on the interaction of chiral molecules and electron spin at different scales; 2) exploring strategies for the enhancement of spin polarization, which is one of the most important issues in developing spintronics materials. The long-term goals are to aid our understanding of the chirality-based spin transport at different scales and reveal principles about how to design and assemble functional artificial systems for spintronics applications.
Representative publications